Subscribe
Sign up for timely perspectives delivered to your inbox.
The biotech industry is experiencing a renaissance, with the US approving a record number of novel therapies in 2018. But not all drugs are created equal, according to members of the Janus Henderson Global Life Sciences team, making it important for investors to focus on both the science and commercial potential of new medicines.
Innovation is a hallmark of biotechnology, and the medical breakthroughs that the industry is now delivering could arguably be called unprecedented. But even as the potential for growth ramps up, we believe investors need to take a prudent approach to investing in the sub-sector.
In 2018, the US Food and Drug Administration (FDA) approved 59 novel therapies, the most of any year on record1. What’s more, the calibre of medicines was noteworthy, with many representing exciting advances for their disease categories, from rare life-threatening disorders to more common ailments.
Consider migraines, the severe headaches that afflict as many as one billion people globally2. For years, migraine drugs treated only the symptoms of the neurological condition. But last year, the FDA approved the first calcitonin gene-related peptide (CGRP) inhibitors. These antibodies work to block CGRP from binding to a receptor, believed to play a key role in the pathology of migraines. In clinical trials, patients treated with the inhibitors saw a significant reduction in the number of days they experienced symptoms. In other words, CGRPs help prevent migraines from developing in the first place, representing a marked improvement in quality of life.
[caption id=”attachment_79486″ align=”alignnone” width=”680″]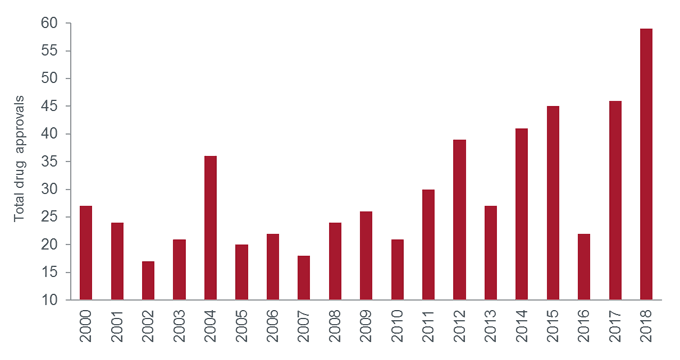 Source: US Food and Drug Administration, as at 31 December 2018. Note: drug totals are for new molecular entities.[/caption]
Source: US Food and Drug Administration, as at 31 December 2018. Note: drug totals are for new molecular entities.[/caption]
Regulators are helping facilitate these advances with faster pathways for drug approvals. The FDA, for one, now offers a breakthrough therapy designation, which expedites the review of medicines that address serious illness or improve the standard of care. The European Medicines Agency recently introduced a PRIME pathway, which aims to reduce review times for novel drugs by nearly a third. Finally, the Chinese Food and Drug Administration has for the first time begun to accept non-Chinese clinical trial data as part of its drug approval process. These avenues not only make it possible to bring drugs to market more quickly, but also reduce the cost of drug development.
As more firms target these accelerated pathways, regulators are bracing for an explosion in advanced medicine applications. In 2019, the FDA is expected to approve AVXS-101, a gene therapy for infants affected by spinal muscular atrophy (SMA). The drug’s approval could be transformational for these young patients: SMA is the leading genetic cause of infant death globally3. It could also become a platform for the manufacture of other gene therapies – a key reason, in our opinion, for Novartis’ $8.7 billion acquisition last year of AveXis, the developer of AVXS-101. To that end, by 2025, the FDA estimates it will be approving 10 to 20 gene and cell therapies annually4.
In addition, other methods for targeting disease are becoming available. Last year, the FDA approved the first RNA interference (RNAi) therapeutic, Onpattro, for the treatment of peripheral nerve disease. RNAi works by using a gene’s own DNA sequence to turn off the expression of a malfunctioning gene. By silencing the sequence, Onpattro could essentially arrest, or even reverse, this painful hereditary condition. Other advanced therapies could have similar effects. For example, gene therapies involve creating a functional copy of a missing or defective gene. Once inserted into cells, the manufactured gene creates a protein that previously may have been missing from a patient, potentially curing the disease. And within cancer, great strides are being made with immunotherapies. These treatments involve disrupting inhibitors that prevent the immune system from recognising cancer cells, or re engineering T-cells (a type of immune cell) to target and kill cancer. Results have been impressive, with one immunotherapy, Keytruda (manufactured by Merck & Co.), reducing the risk of death for the most prevalent form of lung cancer by 50%.
As exciting as these advances are, we believe investors should keep in mind that such progress can be disruptive and faces risk. For one, industry research has found that roughly 90% of compounds that enter human clinical trials never make it to market. In addition, our experience highlights that of therapies that do succeed, investors either over- or underestimate the commercial opportunity about 90% of the time. These odds can lead to dramatic moves in biotech stocks.
With these challenges in mind, we believe investors should be keenly focused on valuation, looking for stocks that trade below the scientific and commercial potential of a company’s products and pipeline. After the market’s pullback last year, we feel a number of biotech stocks offer attractive valuations, potentially leading to a pickup in mergers and acquisitions. Indeed, more than $80 billion of biotech deals were announced in the first month of 2019, highlighted by Bristol-Myers Squibb’s planned acquisition of Celgene.
We also think it is critical to look for companies developing medicines that add value to the healthcare system. The Institute for Clinical and Economic Review, an independent research organisation based in Boston, Massachusetts, is playing an increasing role in evaluating the clinical and economic value of new drugs. We believe companies will need to demonstrate the value of their medicines in order to obtain attractive levels of reimbursement.
This value could come into sharper focus as more countries attempt to balance rising costs, aging populations and increased living standards. In 2017, for example, China updated its National Reimbursement Drug List, which includes therapies approved for reimbursement in the country’s government-run health insurance programme (which covers nearly 100% of China’s population). It was the first update since 2009, and resulted in more than 300 new medicines being added to the list. China also recently approved immunotherapies from Merck and Bristol-Myers. Early data indicate that the launch trajectories for these cancer-fighting drugs in China could rival the rapid uptake in the US. We believe such demand could further fuel biotech’s impressive growth, and help the companies developing high-value drugs stand out.
References:
1https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm592464.htm
2 https://migraineresearchfoundation.org/about-migraine/migraine-facts/
3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5823674/
4 https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm629493.htm
The healthcare industries are subject to government regulation and reimbursement rates, as well as government approval of products and services, which could have a significant effect on price and availability, and can be significantly affected by rapid obsolescence and patent expirations.